Accumulation of DNA damage and telomere dysfunction are well established events in the etiology of cancer, aging and disease. However, until recently, a lack of physiologically relevant model systems prevented our deep understanding of the pathways connecting activation of DNA damage responses to tissue failure and transformation
At the Batista Lab, we utilize the targeted differentiation of human pluripotent stem cells to understand the etiology of disease and cancer after accumulation of DNA damage and exacerbated telomere shortening. We combine biochemical and mechanistic studies with our ability to differentiate human pluripotent stem cells into functional cell types to determine the importance of DNA repair and telomere maintenance in different cell populations and decipher the events that lead from accumulation of DNA damage to disease in humans.
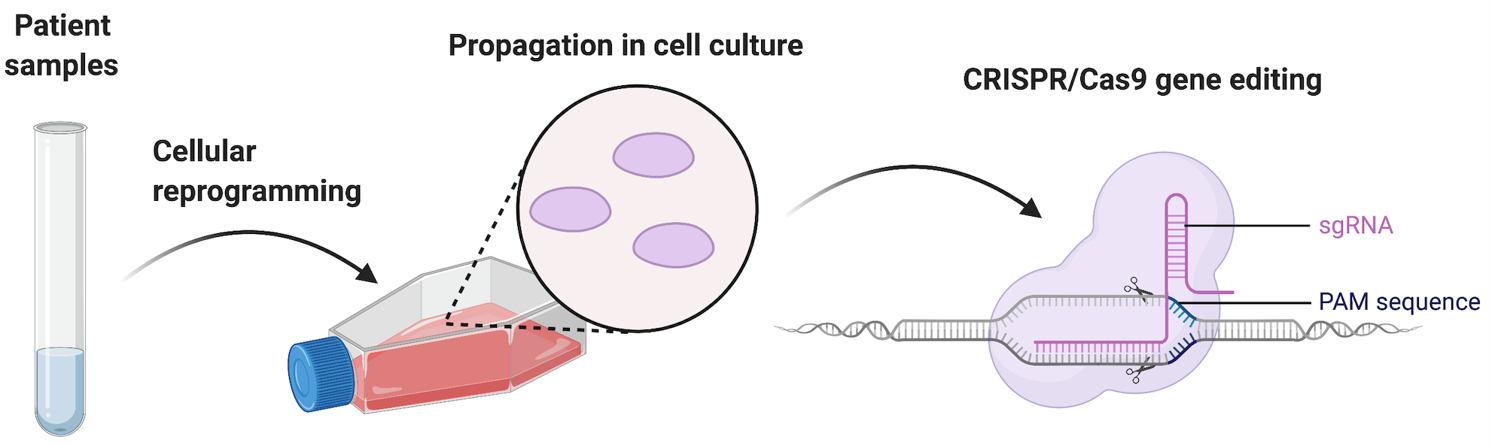
Cellular Reprogramming and Engineering
The study of disease-associated, genetic mutations in telomerase and other DNA repair pathways is complicated by low expression of pathway components in human cells, and the low amounts of relevant cellular populations that can be obtained from clinical samples. Additionally, different biochemical properties between human and mice make the study of patient mutations hard to perform in animal models. To overcome these limitations our lab uses cellular reprogramming from patient fibroblasts with different mutations in telomerase, coupled with genome engineering by CRISPR/Cas9 to create novel, physiologically relevant human pluripotent stem cells to study the consequences of DNA damage accumulation and telomere dysfunction during tissue development.
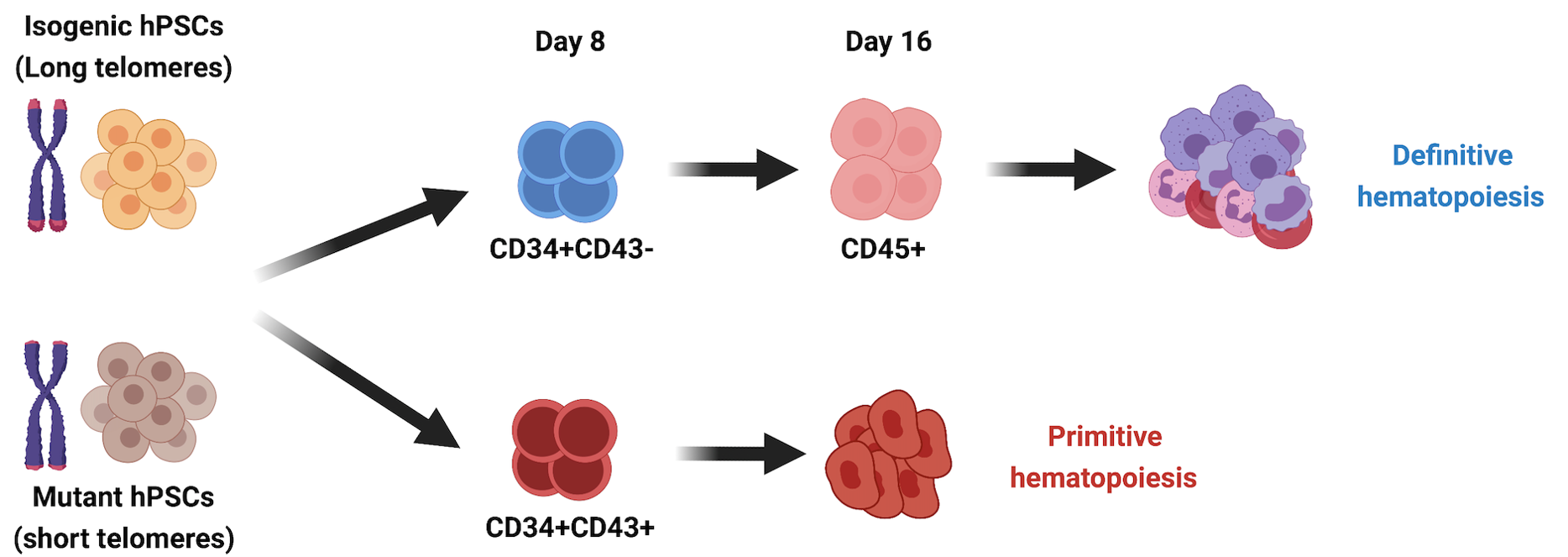
DNA damage and telomere shortening during hematopoiesis
DNA damage accumulation and telomere erosion are associated with hematopoietic failure in different bone marrow failure syndromes, including Fanconi anemia (FA) and dyskeratosis congenita (DC). The progression and molecular determinants of hematopoietic failure in FA and DC remain poorly understood. In the Batista lab, we directly differentiate human embryonic stem cells clinically-relevant mutations to understand the consequences of DNA instability on the primitive and definitive hematopoietic programs. We established hematopoietic failure in DC is restricted to the definitive program and it is caused by DNA damage accrual and is mediated by p53 stabilization. With this model we have created a unique and extremely robust platform for therapeutic discovery for treatment of patients. We are currently pursuing different molecular strategies to achieve this goal, partnered with other collaborators, including Roy Parker’s lab at HHMI/UC Bolder.
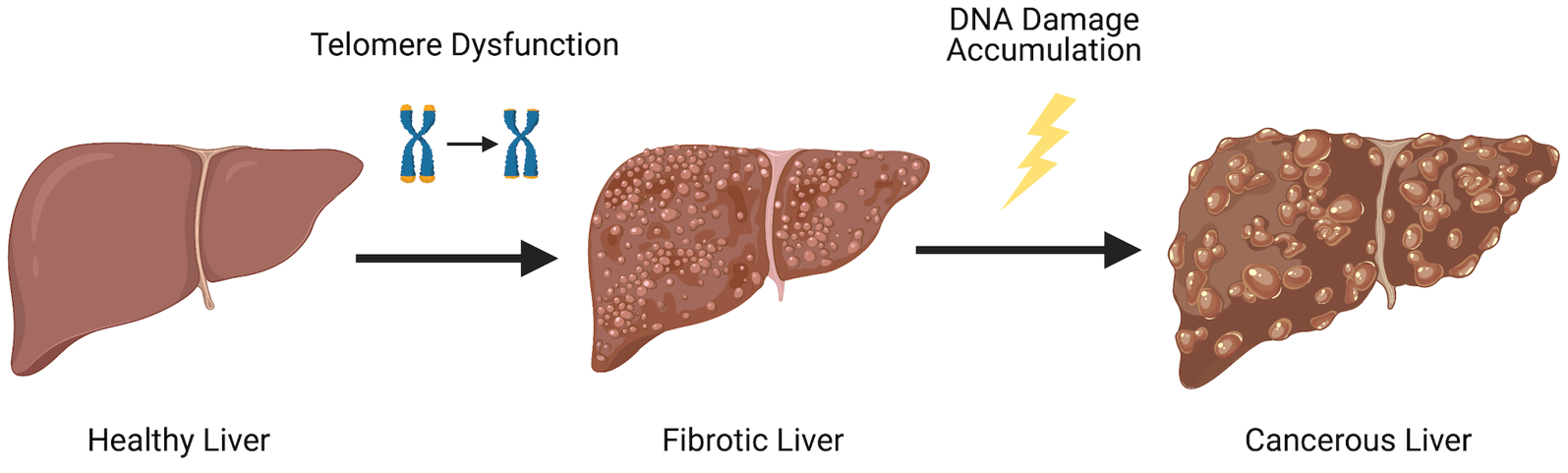
DNA damage accrual in hepatocellular carcinoma
Hepatocellular carcinoma (HCC) is the fifth most common human cancer. More than 80% of HCCs develop from the formation of fibrotic tissue in the liver. It is well established that hepatocytes play a central role in this response. Interestingly, patients with loss-of-function mutations in telomerase are at an increased risk of developing this condition. However, due to lack of adequate models, the role of telomerase and telomere dysfunction has not been rigorously studied in human disease. To circumvent this, we have recently created novel cellular models that allow the identification of the precise mechanisms behind hepatocyte in settings of telomere erosion and accrued DNA damage.